Original Post Published 2019 (see Updates here)
Continuous Walking training with Functional Electric Stimulation (FES) with weight support (where needed) is a KEY to recover standing & walking for many paralyzed patients.
Neuro-plasticity can help only if this program is persistently performed
with a 2 to 3 hours of FES walking practice per day with 5 training’s per week, for at least 6 months.
Key Elements of FES Walking Therapy
- Functional Electric neuro/muscular Stimulation (FES) programmed for walking based on PARASTEP
- Training performed with help of Weight support system for over-ground walking (if needed*)
- Continuous training in length of 3 hours of walking per day, 5 days per week for 5-6 months
- EMS suite or bands to keep electrodes adjusted and fixed to precise positions
If only standing and walking exercise is repeated to number of hours, dates and months, many incomplete Spinal cord injured patients will see benefits and some will even start independently to stand and walk. (explained in Kunming paper and in Tight Rope Walking);
Adding FES to the same continuous standing & walking exercise intensity and length, we can expect even better results.
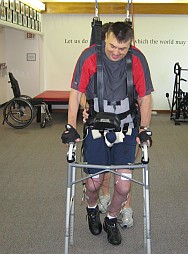
Note: Here is Photo and also Link to YouTube video where you can see that I have already use and tested Parastep shortly in private clinics in USA. and I have witnessed stepping improvement after just few sessions.
If peripheral nerves are intact but due to disrupted communication with brain and spinal cord after injury, chances are that with specific FES technique and due to effort and repetitiveness of correct motion, our brain & spinal cord & the whole nervous system will try to reconnect existing dormant intact nerves and due to neuro-plasticity suppose to establish new neuro pathways to enable certain motion.
Parastep, FES walking device major quality is to provide on demand (push button) type of programmed electric stimulation that will enable muscle contractions to hold stance in one leg while the other leg is stepping forward.
4 channel and 6 channel versions of Parastep were available while this device was in production since 1980-90’s.
6 channel Parastep was also engaging gluteus muscles and was excellent therapeutic walking device for higher spinal cord injuries.
Unfortunately, Parastep was never further developed and vanished from horizon as a rehab option.
Few previous Parastep users are trying to re-create this device and to improve it with new functions and modern electronics.

Weight support system will enable patients to control their stepping and help with repetitions avoiding injury. *Some patients that can already stand and even step independently would never need weight support.
Walking systems with weight support are available from USA but also directly from Chinese suppliers on Alibaba for way lower price.

With the idea of continuous and more frequent use of electric stimulation than in previous Parastep setups, issues with correct stim delivery to avoid skin irritation (eczema) lead also to idea of use EMS suite with continuous supply hydrogel electrodes adjusted and fixed to certain position for easier rehab application. This suite development is still in prototype phase.
Once when FES became available I am planning to organize dedicated 6 months therapy based
on 3 hours daily weight supported walking, 5 days per week and continuously for 6 months along with other strength exercises.
This therapy is something I have envisioned for myself and different modalities to be performed by other people. As all SCI patients are different chances are some will be able to see benefits from less repetitions and some will never be able to recover some functions.
Further Explanations of New Walking Therapy and updates
People with Incomplete Spinal Cord Injury (SCI) can restore walking but only if they actually try to walk again. To begin this process, they need to use special support devices to enable proper walking technique in order to put their body through repetitive walking therapy rounds.
Majority of SCI individuals need “Gait training”, this is based on a body weight support machine. Treadmill and harness system are also part of this package. There are several phases and modes of how walking can be done. This is based on the person’s capabilities to initiate stepping. For many, robot walkers as Locomat and THERA-Trainer lyra or REHA G-EO system are necessary for beginning of this process.

Assistive walking with weight support – Click to see short Video
Some of us that can slightly initiate stepping but lacking intensity and control of movement and need Manual assistance of 2 “walkers” to move legs in rhythmic stepping pattern on treadmill. With this exercise stepping pattern is improving tremendously!/
Another progressive step is addition of Functional neuro-muscular FES stimulation (with or without a weight support) walking training . This type of system is known as Parastep- which was invented around a quarter century ago, and is highly underrated as a therapy tool for Incomplete SCI patients.
The way how Parastep works is simple – when patient try to move the right leg as at the same time press right button on the walker. Small wearable computer send tiny electric stim through 6 electrodes attached on each leg in programmed sequence. This stimulation generate muscle contraction exactly in order to make stepping motion with right leg. At the same time, muscles on the left leg receive signal to straight up and hold body weight on that side.
System Parastep is available from Sigmedics. Parastep is in use by many clinics in the USA. It can be used with and without weight support system that is sold separately.
Parastep, Walking FES as Therapeutic device would be more effective and much faster in application if improved with programmable FES garment.

Maybe the most important conclusion is that no walking therapy for SCI patients can be effective if is not performed in certain (1)frequency, (2)duration and (3)continuance with at least 4-5 trainings per week for 6 months!
Walking therapy is the only way to strengthen weak or to awake unused neuro-circuits and build (again) pattern based neural behaviour that would further support patient walking rehabilitation.
Body-Mind connection, as patient need to highly focus on stepping and pushing, is also crucial element for faster rebuilding of walking neural pattern.
On the other side, failing to perform certain number of repetitions will prevent body to rebuild that neurological pattern.
With above exercises, patients improve very slowly and lose it in no time if stop exercising. This is known as Yo-Yo therapy effect.
Next level of walking exercises has more modalities and different devices that can support patient.
Some people that still need help with stepping might get assistance by robotic devices (Keeogo or Hal or any other full or partial Exoskeleton devices);

Walking therapy is so effective according to Special Hospital for Spinal Cord Injuries in Kunming in China. After decades of experience Specialists in Kunming Chine claim that their method : Continuous walking over ground with high walker and no body weight support in length: 6 hours per day, 6 days per week and 6 months based on “tight rope walking” method – apparently is helping many SCI patients to recover to independent living and walking.
Dr. Wise explain this walking technique on above photo. Research Papers available here.
All above statements, photos and videos from Assisted Gait walking and Parastep use are my own experience and can be repeated and proven
* * *
Some References and Scholar articles related to FES (Functional Electric Stimulation) combined with weight support walking. First if you enter google search for “FES weight supported walking” you will find many results and all researchers agree that this type of physiotherapy has significant advantage over just FES bike therapy and over just weight supported treadmill exercise. (some references 1, 2, 3